The Ice Age of the recent past was a fascinating time, full of superlative animals, especially the mammalian megafauna of North America. The Ice Age, also referred to as the Pleistocene epoch, lasted from 1.9 million years ago to 10,000 years ago, and was characterized by a series of glacial advances and retreats across much of the Northern Hemisphere. It was also a time of animal migrations between continents and of many species being exceptionally large.
Giant ground sloths, the giant short faced bear, saber-toothed cats, mammoths, and mastodons all tromped through what was to later become our backyards. Many people are surprised to learn that North America was also home to a very large cat, larger than the modern lion, given the scientific name Panthera atrox.
This big cat lived mostly across the western half of North America, and ranged into South America as far as Peru. Its remains are plentiful in the tar pits of Rancho La Brea. It is clear that this is a big animal. Estimates of body size suggest a weight of about 1,000 pounds, and that it stood 4 feet at the shoulder. For comparison, the modern African lion weighs in at about 375 pounds. This American cat would have been the second largest mammalian predator, right behind the giant short faced bear. (See How big was the Giant Short-faced bear?)
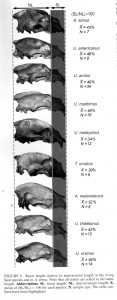
However, understanding how this animal relates to other large cats has been difficult. Scientists have noticed similarities between P. atrox and the modern lion, tiger, and jaguar. For many years, P. atrox was thought to be a subspecies of the lion, and so it has popularly been called the American Lion, and even the American Cave Lion. If it is closely related to the African lion, it suggests that lions migrated out of Asia and into the New World during the Ice Age, expanding as far south as South America, and becoming extinct at the end of the age. Several other species are known to have done this, so that is not so unusual. But is it an accurate story?
In a recent paper on the subject (Christiansen and Harris 2009), researchers have come up with a different idea. They examined the skull and jaws of the big American cat and compared it with lions, tigers, and jaguars. They used a wide range of measurements to create a mathematical model of each species, and compared them to each other. The result? Panthera atrox does not seem to be a lion at all, but rather is closest to the modern jaguar.
Jaguars came into the New World from Asia during the early Pleistocene. It seems then that P. atrox and the modern jaguar species, P. onca, are derived from the early jaguar that came into North America, and that lions never made that long trek across. If these researchers are correct, we should not call this magnificent cat the American Lion.
So, what should we call it? Jaguars are native to the New World, so the word “American” seems a bit redundant in the name. And the simple scale and grandeur of the cat requires some adjective. “Mega Jaguar” seems a bit plain to me. What do you suggest?
Christiansen, P. and Harris, J. M. 2009. Craniomandibular morphology and phylogenetic affinities of Panthera atrox: implications for the evolution and paleobiology of the lion lineage. Journal of Vertebrate Paleontology 29(3):934-945.